Peanuts are one of the most common allergens, causing very severe allergic reactions. Peanut allergy accounts for 59% of the total number of food allergies and affects approximately 3 in every 100 children. The exact cause of allergy is not clear, but at least 11 specific proteins are recognized as peanut allergens. The allergen proteins can be made hypoallergenic after heating, which is called desensitization treatment. Essentially, this treatment can make modifications in protein structure, and the body no longer recognizes it as a pathogen, thus avoiding the allergic reaction.
The research group of Prof. Hengchang Zang at School of Pharmaceutical Sciences, Shandong University is dedicated to unraveling the relationship between the structure of proteins and their functionality, using aquaphotomics. Among many other biomolecules, this laboratory also explored the changes of the peanut allergen during heating process. In a recent publication, “Research on the Structure of Peanut Allergen Protein Ara h1 Based on Aquaphotomics” they applied aquaphotomics, using water as the probe to explain the detailed structural changes of peanut allergen protein Ara h1 during the heating process.
The first author of the article, published in June issue of Frontiers in Nutrition, is young Miss Mengqi Zhang, who is interested in applying Aquaphotomics to understand the structural information of natural products that could affect its function.
Aquaphotomics processing tools including principal component analysis (PCA), continuous wavelet transform(CWT), and two-dimensional correlation spectroscopy (2D-COS) were utilized for better understanding the thermodynamic changes, secondary structure, and the hydrogen bond network of Ara h1. The results showed that about 55 oC could be a key temperature which was the structural change point. During the heating process, the hydrogen bond network was destroyed, free water was increased, and the content of the protein secondary structure was changed. This discovery revealed the interaction between the water and Ara h1 from the perspective of water molecules and explained the effect of temperature on the Ara h1 structure and hydrogen-bonding system. Thus, it described a new way to explore the thermodynamic properties of Ara h1 from the perspective of spectroscopy and laid a theoretical foundation for the application of temperature-desensitized protein products.
The research was published June 18, 2021 in Frontiers of Nutrition, as a part of the research topic The Future Food Analysis. (https://doi.org/10.3389/fnut.2021.696355)
This research showed that during the heating process, the water spectra of the Ara h1 aqueous solution gradually shifted from high wavelength to low wavelength (Figure 1). The structures of Ara h1 and its spectra were changed under the influence of temperature. During the heating process, the strong hydrogen bonds were destroyed gradually, weak hydrogen bonds and other structures were formed. Under the influence of this effect, the water molecules were bounded by weak hydrogen bonds increasing. As the temperature rose again, the weak hydrogen bonds were also destroyed, the absorbance was weakened there, and the free water structure was formed. When the temperature was below 55oC, the WAMACS of the aquagram was mainly biased towards high wavelengths. At this time, the hydrogen bond network structure of Ara h1 aqueous solution was stable, and the hydration was strong between water and the protein surface, which had little effect on the protein structure. When the temperature was higher than 55oC, the aquagram tended to be at low wavelengths, the hydrogen bond network was broken, hydration was weakened, the structure of Ara h1 aqueous solution was greatly changed, and the protein precipitated and aggregated, and the hydrophobicity of the protein increased. It led to the decrease of sensitization ability.
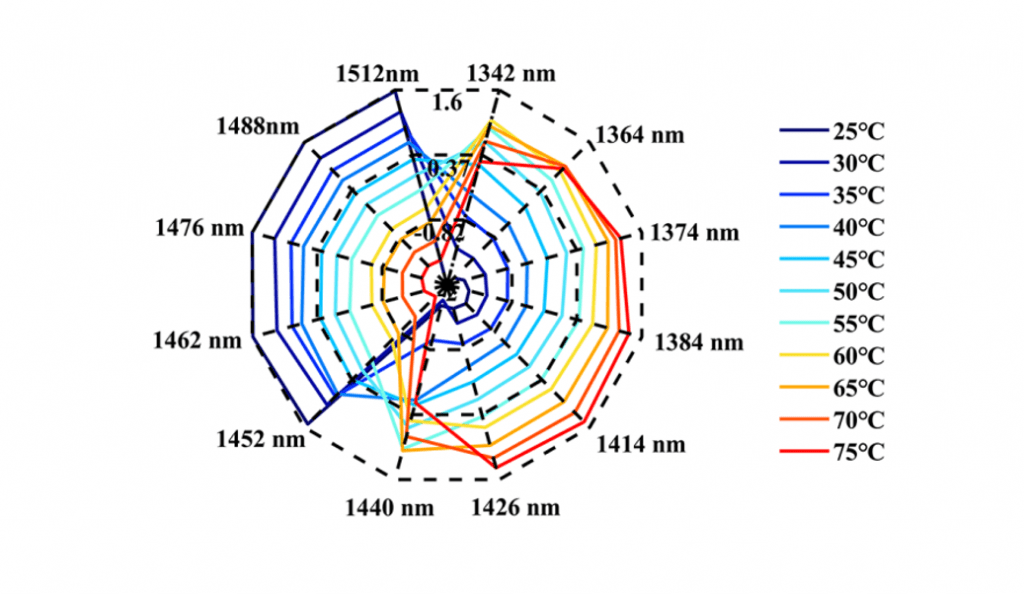
The structures of Ara h1 had undergone major changes at around 55 oC, causing a rapid increase in the β-sheet content of the amide A /III (Figure 2 and Table 1). The α-helical content had been rising during the heating process. The secondary structures of the protein changed, and the side chain structures of the protein were broken, resulting in corresponding decreases of the sensitization ability of Ara h1.
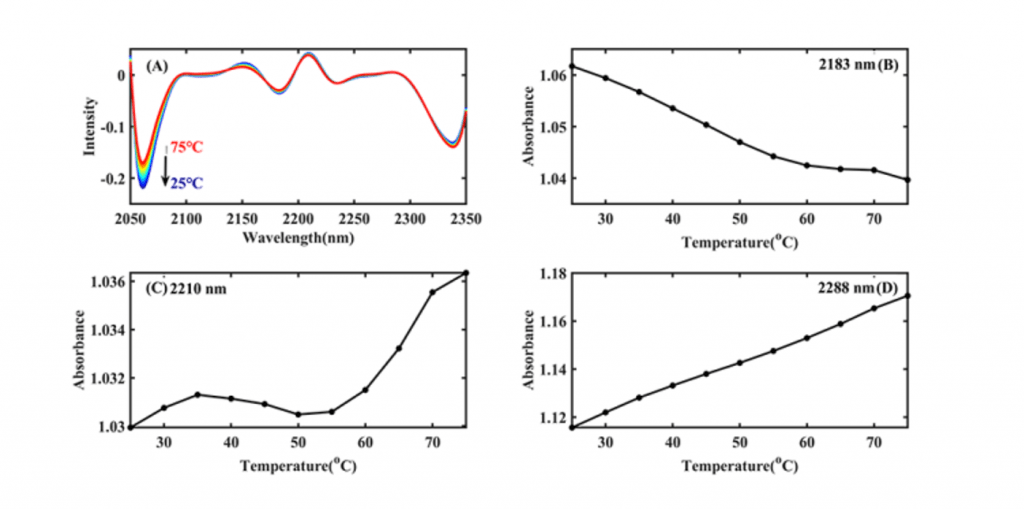
Table 1 Characteristic absorption of Ara h1 aqueous solution in NIR spectra
| number | Wavelength (nm) | characteristic absorption band |
| 1 | 2060 | the N-H bending vibration; the second overtone of an -OH bending vibration of water |
| 2 | 2183 | Amide B / II |
| 3 | 2210 | β-fold |
| 4 | 2288 | α- helix |
| 5 | 2342 | -CH2 side chain |
A method for characterizing the regularity of protein structural changes without labeling was established through this research. Compared with the conventional analysis method for structural changes, it was easy to operate and had high sensitivity. At the same time, it was based on the interaction between the allergen protein Ara h1 and the water structure, revealed the temperature point of the Ara h1 protein structural changes. It laid a theoretical foundation for food processing technology, and also provided a new idea to explore the interaction of various molecules in the life system.
For more information about this research, please contact the corresponding authors.
Mengqi Zhang
First Author
School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, China
Hengchang Zang
Corresponding author
School of Pharmaceutical Sciences, Shandong University, Jinan, China
zanghcw@126.com
Lian Li
Corresponding author
School of Pharmaceutical Sciences, Shandong University, Jinan, China
lilian@sdu.edu.cn
